Next Lesson - The Endocrine Pancreas
Abstract
- The thyroid gland lies anterior to the larynx and trachea, inferior to the thyroid cartilage, with the isthmus extending from the 2nd to 3rd rings of the trachea.
- The thyroid gland consists of follicular cells which produce thyroglobulin, and parafollicular cells which produce calcitonin.
- Thyroglobulin acts as the scaffold to produce thyroid hormones T3 and T4, and iodine is essential for the formation of these molecules.
- Thyroid hormone production is regulated via negative feedback.
- Thyroid hormones affect a range of metabolic pathways.
- Goitre is a swelling of the thyroid gland, and can be physiological or pathological.
- Hyperthyroidism is characterised by high T3 and T4 and low TSH, whilst hypothyroidism is characterised by low T3 and T4 and high TSH. Both of these conditions have wide-reaching effects on the body.
Core
Thyroid Anatomy and Development
The thyroid gland lies anterior to the larynx and trachea, and inferior to the thyroid cartilage. The isthmus (the part of the thyroid gland that joins the two lobes together) extends from the 2nd to 3rd rings of the trachea. The thyroid gland is shaped like a bowtie.
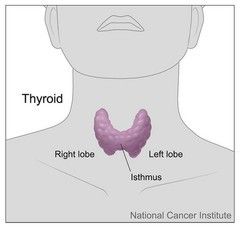
Image: Shows the position gross anatomy of the thyroid gland
Creative commons source by Don Bliss (illustrator) [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
The thyroid gland first appears as an epithelial proliferation on the floor of the pharynx between 3-4 weeks gestation. In order to migrate to its final position, it descends as a diverticulum (an outpouching of tissue) through the thyroglossal duct anterior to the hyoid bone. The thyroglossal duct connects the thyroid gland to the tongue, but by birth this duct should have degenerated.
Failure of this duct to degenerate can lead to conditions such as a thyroglossal duct cyst, seen as a lump on the midline of the neck. The lump moves upwards on tongue protrusion because the duct is still connected to the base of the tongue.
It is important to remember that the thyroid gland and the parathyroid glands are two separate glands. The parathyroid glands are usually four small glands around the thyroid, however this number can vary between individuals. They contain parathyroid principal cells (also known as chief cells), which produce parathyroid hormone, an essential hormone for calcium homeostasis. The parathyroid gland will be further discussed in the ‘calcium homeostasis’ article of the endocrinology series.
Thyroid Structure and Function
The thyroid gland consists of follicular cells and parafollicular cells. The follicular cells are arranged in spheres called thyroid follicles and produce thyroglobulin. Thyroglobulin is stored outside the cell as a substance called colloid which surrounds the cells and acts as the source of tyrosine residues needed for the production of thyroid hormones. Colloid is classed as an extracellular substance, even though it is within the thyroid gland, as it surrounds the follicles. The parafollicular cells produce calcitonin which is essential for calcium homeostasis.
The Production and Regulation of Thyroid Hormones
Thyroid hormones (and their precursors) are the only molecules in the body that contain iodine (meaning the thyroid gland contains 90-95% of the body’s iodine). Dietary iodine, most commonly found in fish and dairy, must be reduced to iodide (the ion I-) before it can be absorbed into the small intestine. It is then transported through the blood to the thyroid and taken up by thyroid epithelial cells through a sodium-iodide symporter (2Na+ are transported out of the thyroid cells in exchange for one I-). Iodine deficiency is currently a problem in the UK and is discussed later in this article.
The two main thyroid hormones are called triiodothyronine (T3) and tetraiodothyronine (thyroxine – T4). Thyroglobulin produced by the thyroid gland acts as a scaffold for the formation of these hormones. The two hormones are made though the linkage of two molecules of iodinated tyrosine on the thyroglobulin scaffold, with the iodine being located at positions 3 and/or 4 on the aromatic ring of tyrosine depending on T3 or T4 synthesis.
The basic steps of T3 and T4 synthesis are outlined below:
- Thyroglobulinis synthesised in the follicular cells and secreted through exocytosis into the lumen of the thyroid follicle.
- Iodide is transported into the thyroid epithelial cells through the2Na+:I- transporter, against its concentration gradient, and is secreted into the lumen of the thyroid follicle.
- Iodide undergoes oxidation to produce an iodinating species, catalysed by thyroid peroxidasein the presence of H2O2.
- Side chains of tyrosinefound in the thyroglobulin are iodinated to form either mono- or diiodotyrosine (MIT or DIT), catalysed by thyroid peroxidase.
- MIT and DIT then couple to formT3 and T4 within the thyroglobulin, catalysed by thyroid peroxidase:
- MIT + DIT → T3
- DIT + DIT → T4
- Thyroglobulin, with the newly formed thyroid hormones attached to its backbone, is brought back into the thyroid follicular cells via pinocytosis.
- Thyroglobulin is broken down and the thyroid hormones are released. Any uncoupled iodine residues are recycled.
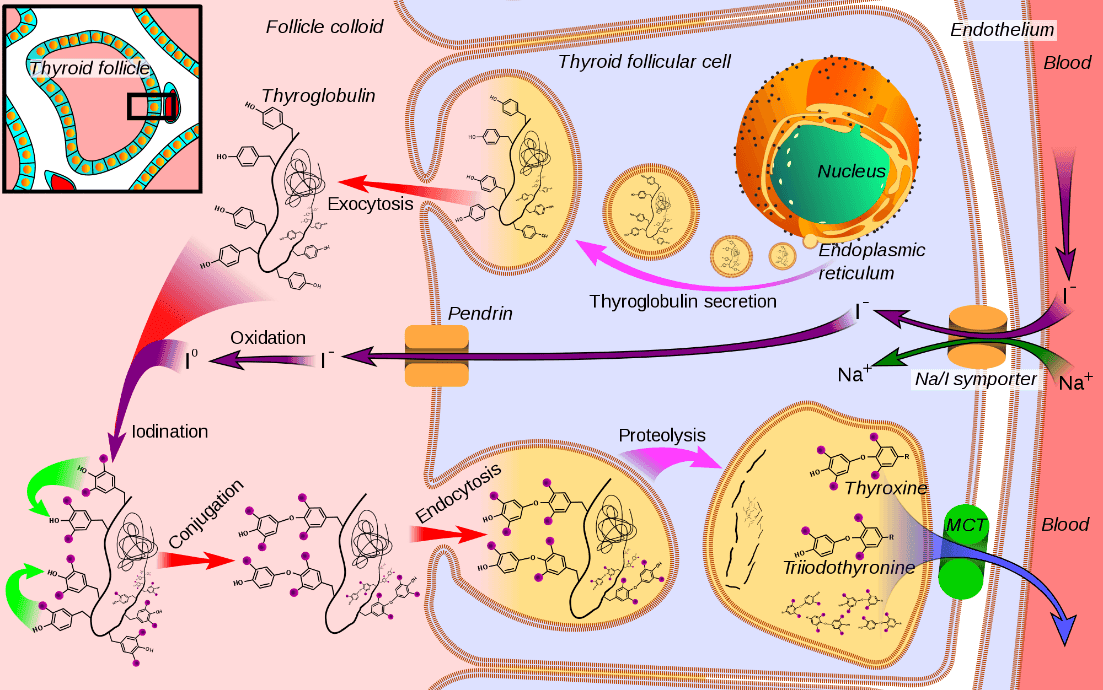
Diagram: Shows thyroid hormone production: thyroglobulin is synthesised and exocytosed into the collide (top left). Iodide is transported into the collide to join with tyrosine on the thyroglobulin (left). The resulting MITs and DITs are conjugated to form T3 or T4 attached to thyroglobulin (bottom left). The thyroglobulin is taken into the cell and undergoes proteolysis to liberate the thyroid hormones which are released into the blood (bottom right).
Creative commons source by Mikael Häggström [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)]
90% of thyroid hormone secreted is T4. However, T3 is four times as biologically active, meaning most T4 is converted to T3 by the liver and kidneys. Thyroid hormones are hydrophobic, so both T3 and T4 are transported in the blood bound to thyroxine-binding globulin.
Thyroid hormone secretion is regulated via negative feedback, outlined in the diagram below.
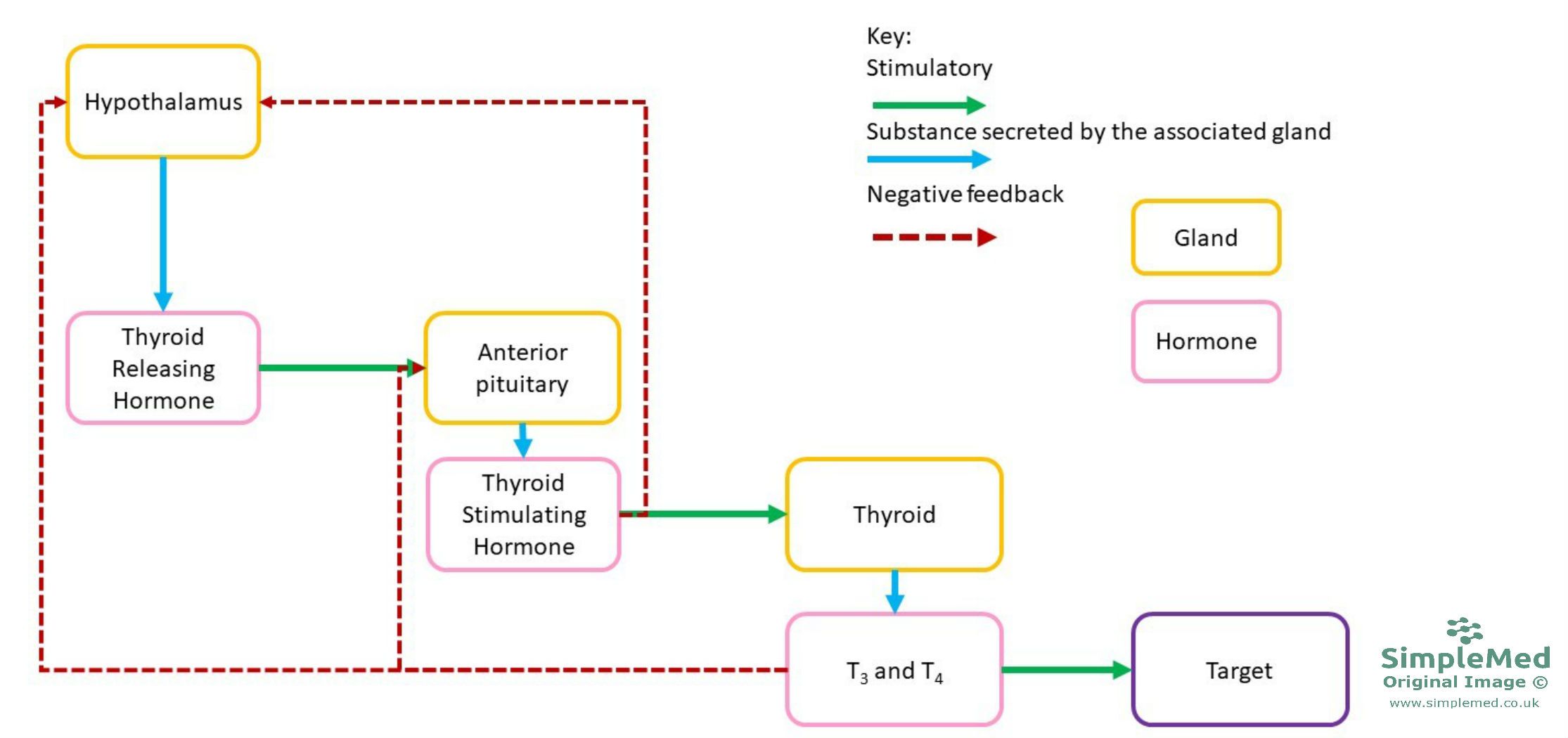
Image: Shows the regulation of thyroid hormone production
SimpleMed original by Jenny Hubball
Thyroid-stimulating hormone (TSH) is a glycoprotein hormone composed of 2 non-covalently bound subunits, one α and one β. The α-subunit is the same subunit that can also be found in FSH and LH, meaning the β-subunit is what makes it unique. TSH stimulates a range of processes using GPCRs (Gα-s and Gα-q). The processes that TSH stimulates are listed below:
- Iodine uptake.
- Iodine oxidation.
- Thyroglobulin synthesis.
- Thyroglobulin iodination.
- Colloid pinocytosis.
- Thyroglobulin proteolysis.
- Thyroid cell metabolism and growth.
Thyroid hormone receptors are nuclear receptors, also called hormone response elements, that are pre-bound to the DNA in the promotor region of thyroid-hormone-regulated genes. In the presence of thyroid hormones, these receptors undergo a conformational change and are activated to become transcription factors which modulate gene expression. When no thyroid hormone is present, the receptors bind to DNA and cause transcriptional repression.
Examples of thyroid-hormone-regulated genes are PEPCK, Ca2+ ATPase, Na+- K+ ATPase, cytochrome oxidase, and 6-phosphogluconate dehydrogenase. These hormones are linked to the effects of thyroid hormone outlines below.
Thyroid hormones affect virtually every cell in the body, with their main responses having effects on cellular differentiation and development, and metabolic pathways. Some of the main functions are outlined below:
- Increase basal metabolic rate andheat production in tissues by increasing the size and number of mitochondria and stimulating the synthesis of enzymes in the respiratory chain. Tissues which are not affected in this way include the brain, spleen, and testes.
- Stimulate metabolic pathways(mainly catabolic).
- Stimulate lipolysis and β-oxidationof fatty acids.
- Stimulate insulin-dependent entry of glucoseinto cells.
- Increasing gluconeogenesisand glycogenolysis.
- Increasing the number of adrenoreceptors on cells, therefore having a sympathomimetic
Pathology of the Thyroid Gland
Goitre
A goitre is an enlargement of the thyroid gland, developing when the thyroid gland is overstimulated. A goitre may accompany hypo- or hyperthyroidism but is not always present in either condition. A physiological goitre can occur during menarche, pregnancy, and the menopause.
The most common causes of goitre are:
- Iodine deficiency, which leads to reduced thyroxine levels (hypothyroidism) and therefore increased TSH release due to the feedback loop. The increased TSH causes thyroid cell maturation and proliferation, increasing the size of the gland and causing a goitre.
- Multinodular goitre, the cause of which is currently unknown. This is the most common cause of a goitre in the UK. After a number of years this may become toxic multinodular goitre, causing the patient to develop hyperthyroidism. A multinodular goitre may also enlarge inferiorly into the superior mediastinum to form a retrosternal goitre. This may cause tracheal compression.
It is possible (but rare) for thyroid nodules to be malignant but thyroid cancers often do not cause a metabolic disturbance.
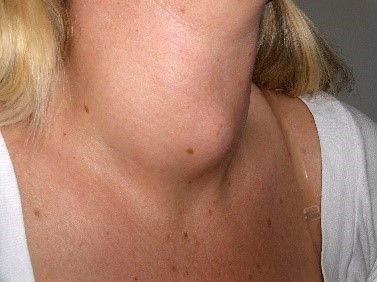
Image: Shows a goitre (an enlarged thyroid gland)
Creative commons source by Drahreg01 [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)]
Hypothyroidism
Hypothyroidism can have many causes, including:
- Failure of the thyroid gland
- TSH or TRH deficiency
- Inadequate dietary supply of iodine
- Radioactive iodine intake
- Autoimmunity – Hashimoto’s disease
- Post-surgery complications
- Congenital defects
- Anti-thyroid medication
The general symptoms of hypothyroidism are obesity, lethargy, intolerance to cold, bradycardia, dry skin, alopecia, hoarse voice, constipation, and slow reflexes. Clinically, a patient with hypothyroidism would have low T3 and T4 but elevated TSH in their blood plasma. TSH is elevated due to the lack of negative feedback from the thyroid hormones (think about the diagram pictured earlier).
In infants, severe hypothyroidism can cause cretinism, where the child has a dwarfed stature, mental deficiency, poor bone development, slow pulse, muscle weakness, and GI disturbances. This is commonly due to low iodine intake by the mother during pregnancy. In adults, severe hypothyroidism can cause myxoedema, characterised by thick puffy skin (especially in the shins, called pre-tibial myxoedema), muscle weakness, slow speech, mental deterioration, and intolerance to cold.
The normal treatment for hypothyroidism is oral thyroxine.
Hashimoto’s disease is an autoimmune disease resulting from destruction of the thyroid follicles, leading to hypothyroidism. It is the most common thyroid gland disease and is much more common in women than men. The treatment for Hashimoto’s disease is oral T4, given preferentially over T3 due to its longer half-life even though T4 is less biologically active.
Hyperthyroidism
Hyperthyroidism also has many causes, including:
- Autoimmunity – Graves’ disease
- Toxic multinodular goitre
- Solitary toxic adenoma
- Excessive thyroid hormone therapy
- Drugs (e.g. amiodarone)
- Thyroid carcinoma
- Ectopic thyroid tissue – this can occur especially in the thyroglossal duct.
The symptoms of hyperthyroidism are weight loss, irritability, heat intolerance, tachycardia, fatigue, increased bowel movements, tremor, hyperreflexia, breathlessness, loss of libido, and sweating. Another sign of hyperthyroidism can be staring eyes, in which the levator palpebrae superioris muscle is over-stimulated by the sympathetic nervous system, leading to the eyelids being drawn up causing ‘staring eyes’ and lid lag. Thyrotoxicosis is the term used to describe the clinical effects experienced by the patient due to hyperthyroidism. The clinical markers of hyperthyroidism are elevated T3 and T4 and low TSH blood plasma levels.
The treatment for hyperthyroidism is antithyroid medication, which act by ‘blocking’ the formation of thyroid hormone. This can be done through Carbimazole, an anti-thyroid drug that needs to be taken continuously, or in severe cases, by taking radioactive iodine, which kills off the thyroid tissue and puts the patient into clinical hypothyroidism (this is more desirable because it can be easier to manage).
Graves' disease is an autoimmune disease caused by the production of thyroid stimulating immunoglobulin (TSI), which continually stimulates thyroid hormone secretion outside of normal hormonal control, resulting in hyperthyroidism.
Toxic adenoma is a single adenoma which has developed in the thyroid and produces thyroxine.
TSH levels can be used as a screening tool for hypo-/hyperthyroidism, as it is very rare for a pituitary adenoma to directly produce TSH.
A very high amount of metabolic thyroid disease is due to a primary abnormality of the thyroid itself and not a problem with TSH. If TSH is raised, it indicates that not enough thyroid hormone is being produced, and if TSH is reduced is indicates that too much thyroid hormone is being produced. This is due to the negative feedback loop, where TSH concentrations are modified to control the output of the thyroid gland.
Thyroid scintigraphy is the imaging process used to view the thyroid gland. Technetium-99m is used for isotope scanning of the thyroid with a gamma camera, as it is taken up by the thyroid gland. It is the most commonly used medical radioisotope and has a biological half-life of 1 day, meaning the radiation exposure is low. It can also be used for bone scans, myocardial perfusion imaging, and brain imaging.
Edited by Bethany Turner and Dr. Maddie Swannack
- 8020

